Since its discovery, platelet-rich plasma, or PRP, has developed into a $40-billion-a-year industry and has expanded from oral and maxillofacial surgery and implantology to other medical fields such as dermatology, orthopedic surgery, sports medicine, plastic surgery, and veterinary medicine. As the industry has grown, commercial interests and a lack of standardized language have led to a slew of similar and confusing acronyms: PRP, PRF, A-PRF, i-PRF, PVRG, PRFM, CGF, PC, hcPRP. . . How many of these have you heard of, and do you know the difference between them? Arun K. Garg, DMD, tells us how we got here.
In the Beginning There Was PRP
We expected healing to be a little better. What we found, though, is that healing was a lot better.
After that first success, they began implementing it more, using the equipment already present in the hospital operating room. “At that time, the heart pump we were using to centrifuge was about $80,000,” Dr Garg recalls, “but we didn’t have to buy it. It was already there in the hospital for cardiothoracic surgeons, so we used it. After we applied the material, the bone became sticky and much easier to handle.”
While the improvement to graft handling was immediately apparent, Dr Garg and his team were also eager to see whether this change would result in any improved healing. “We expected healing to be a little better because there would be less migration of the bone material,” he says. “What we found, though, is that healing was a lot better than expected. The healing time was almost twice as fast and the bone growth almost twice as dense. It was drastically better.”
Dr Garg emphasizes that to confirm results like this, he and Dr Marx would normally do several years of work and research before publishing. However, one of their residents at the University of Miami, Pairot Tayapongsak, published on it in 1994 and called the material autologous fibrin adhesive. (He did not use an acronym in this paper.)
“By referring to the material as autologous fibrin adhesive, Dr Tayapongsak put emphasis on the fibrin component of the material,” Dr Garg explains. “And the fibrin content did explain part of the improved healing by way of holding the bone material together, but it didn’t explain the whole result. What we discovered was that the effect wasn’t just due to the fibrin content: it was also influenced by the concentrated platelet content.”
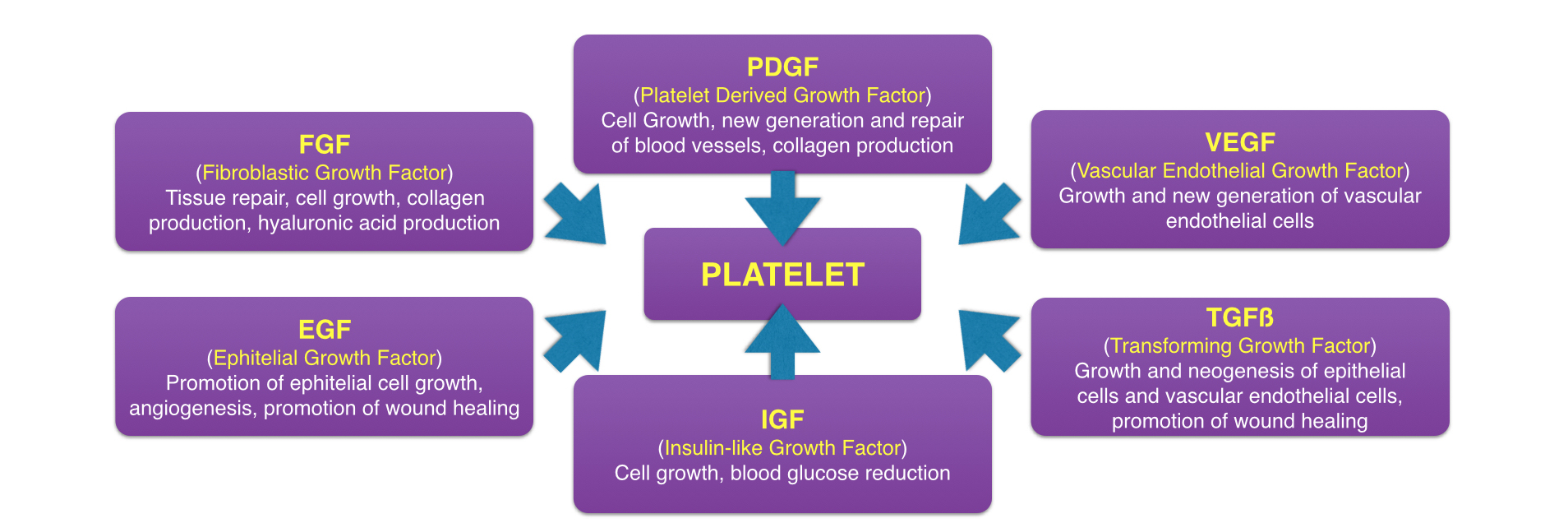
At that time, medical professionals were well aware of the fact that platelets control the first step of healing by being the first to arrive at the site of injury and inducing clotting. However, what was not well known at the time is that platelets also initiate the second step of healing—wound repair—by releasing growth factors at the area of injury. So when Drs Marx and Garg’s team increased the number of platelets to the surgical area by fivefold, they also inadvertently increased the number of platelet-derived growth factors by fivefold. By amplifying these growth factors, they effectively amplified wound healing at the surgical site, resulting in both increased speed and quality of healing.
All of this brings us back to the name. “After this discovery,” Dr Garg said, “we decided that, while fibrin was important for its mechanical impact, it wasn’t the star of the show. So we switched gears to give star billing to the platelets by calling it platelet-rich plasma, or PRP.”
Now that they understood why the PRP was having the effect it was having, Dr Garg and his team began experimenting with different surgical configurations of the material. The original configuration involved adding anticoagulant to the drawn and centrifuged blood to keep it from clotting before use and then inducing clotting once the material was added to the bone graft material. This became known as PRP liquid and can be used in long surgical procedures or as an injectable. The next configuration they experimented with was allowing the PRP to clot before use. They found that the clotted PRP was best applied during short surgeries because, if the material is left to clot for too long, too many of the growth factors are released prematurely and left behind in the receptacle when the material was removed and used.
“The clotted material can’t be used to hydrate the bone,” Dr Garg says, “but you can certainly add it into an extraction socket. This became known as PRP gel. You can also flatten the clotted PRP into a fibrinous membrane and use it to contain bone graft material, and this became known as a PRP membrane. We also published on using the gel in sinus lifts for bone formation: gel on one side, bone on the other. On the crestal side, it can be hard to get bone graft material into that small opening without tearing the sinus membrane. But with PRP gel, you can achieve the necessary stability, and it’s easier to pack it in. The gel will either push up on the sinus membrane or come back out of the osteotomy, but it doesn’t have enough ‘body’ to actually tear the sinus membrane. Before PRP gel, a crestal sinus lift was a very technique-sensitive procedure; however, now it’s ubiquitous.”
All of these were names for the same substance, just in different configurations for different applications, and its versatility as an autogenous material that could be used to enhance wound healing caused it to spread rapidly.
The Market Success and Spread of Autologous Blood Concentrates
After the success seen in oral surgical applications, the use of PRP quickly spread from dentistry to medicine, particularly in specialties like orthopedic surgery, sports medicine, and veterinary medicine. In sports medicine and orthopedic surgery, the use of PRP achieved high demand as an adjunctive treatment for sport-related injuries in professional athletes, as the decreased healing time was especially valuable in getting athletes off the bench and back onto the field. Similarly, PRP spread to veterinary medicine and was used in injured racehorses. The cost of PRP treatment was an easy choice for an athlete with a multimillion-dollar season contract or in a horse like recent Triple Crown winner Justify, who is worth an estimated $75 million. But in order for PRP to become the powerhouse of an industry it is today, the technology needed to be financially accessible for a majority of health providers, and an $80,000 price tag for a centrifuge was far too steep for in-office use.
When Drs Marx and Garg first began using PRP, they were using equipment already available at the hospital where they were performing their surgeries, so there were no start-up costs for implementing the technology—just the operation costs of test tubes and paying a perfusionist to operate the centrifuge during the surgery. Once the use of PRP began to spread, a company called Harvest Technologies released an in-office centrifuge machine that cost just $8,000—a fraction of the price of the original centrifuge Drs Marx and Garg had started using in the hospital operating room. However, there was a special catch to their business model: in order to turn a profit while selling the centrifuge below market value, they cranked up the cost on the test tubes to $350 apiece. The amount spent on single-use test tubes adds up quickly, and, by holding the customer hostage by way of limiting which test tubes could be used in the machine, the company also ensures a longer tail of revenue from each customer. Harvest Technologies put its centrifuge machines in almost every major hospital in the country because they knew that once surgeons started using PRP, they would have to keep buying the test tubes. And it worked: in just 8 years, a Japanese group purchased Harvest Technologies—a company with a single product, the centrifuge and its test tubes—for $80 million.
“After the sale of Harvest Technologies,” Dr Garg explains, “everyone started thinking about how they’d like to make $80 million off one product, so the market exploded. In the US alone, there are probably 40 different manufacturers of these centrifuges now. We started seeing centrifuges for $4,000, $2,000, and $800 with test tubes for $200, $100, and $40 each. The prices were all over the place for the same technology. It was similar to the Keurig model: make the brewing machines inexpensive enough to become ubiquitous, then rely on the higher profit margin of the single-use coffee pods. And everyone says, ‘Our coffee tastes better.’ There have even been companies who try to sell $40 test tubes over $0.30 test tubes by saying—I kid you not—’my test tubes are made of Moroccan glass.’ Who cares if it’s made from Moroccan glass? It’s a test tube!”
So with all the flashy options available, which machine does the original co-discoverer of PRP use? “In our lectures,” Dr Garg says, “we demonstrate the machine by whichever company sponsors the course because the technology is all the same. But the one I use daily in my six practices is a $1,500 machine with generic $2 test tubes. If I were to use a machine with more expensive test tubes, that cost would get passed on to my patients. The cost of a $1,500 machine is nominal when spread out in a practice, so with my setup the cost of PRP treatment is just $2 for the test tube and the needle to draw the blood.”
What’s in a Name? The Many Terms for Autologous Blood Concentrates
As the market became saturated with centrifuge options, a new strategy to differentiate products and capture market share emerged: giving the material produced a different name.
“The first second-generation autologous blood concentrate was PRF, which stood for platelet-rich fibrin,” Dr Garg explains. “The term PRF was coined by a Moroccan-French doctor who also went on to coin other terms such as leukocyte-platelet–rich fibrin (L-PRF), advanced PRF (A-PRF), and injectable PRF (i-PRF), and later A-PRF+ and i-PRF+. A Spanish oral surgeon coined the term plasma rich in growth factors (PRGF), and in Italy and Korea they began calling the material concentrated growth factors (CGF). So now we have all of these terms in an industry that lacks standardization of nomenclature, and clinicians and patients alike are understandably confused.”
So is there a difference between all of these materials?
“The first and most obvious difference is whether or not you use an anticoagulant,” Dr Garg explains, “because that affects the clotting and centrifugation of the material. By instruction, PRF is described as never using an anticoagulant, and because of that much of the literature today states that the major difference between PRP and PRF is anticoagulant inclusion. However, when we first started using PRP, we often forewent the use of an anticoagulant—the decision was always based on the particular application and how quickly we were using the material. So to say PRP is always used with an anticoagulant would be completely inaccurate. We added the anticoagulant because we found that, for lengthy surgical procedures where the material may be drawn long before its use, if we allowed the material to coagulate for too long, too many of the platelet-derived growth factors would be released early in the receptacle rather than at the surgical site.”
“The other differences,” he continues, “relate to the centrifuge itself. By altering the rotations-per-minute (RPM) of the machine, you can affect the concentration. Also important, however, are the size of the rotator arm, how long you spin the material for, and the angle of the test tube in the centrifuge. One thing that you absolutely cannot do is take the production recommendations from one manufacturer’s centrifuge, apply it to another, and expect a good result, because manufacturers set their speed and length of time recommendations based on the specifications of that machine’s rotator arm and angle of the test tube.”
According to marketers, you can only make certain materials using their specific centrifuges. But according to Dr Garg, you can customize the centrifuge process to create any of these materials. “By modifying spin speeds and time, different components of the middle layer can be slightly increased or slightly decreased in concentration. What the clinician must do is identify which of the configurations of autologous blood concentrates will have the effect he or she desires in each individual case. For example, perhaps in one case soft tissue healing is your main concern and increased growth factors are your focus, so you create and apply a PRP gel membrane. In another case, you’re performing a ridge augmentation where the mechanical benefit of increased fibrin content would be clinically appreciated, so you create and apply a clotted PRP membrane. Each configuration has its set of preferred applications, and you have to be able to identify which configuration is needed in each case in order to achieve the best possible result.”
But with so much contradictory information out there, how do clinicians parse through the advertising narratives from manufacturers to find the instruction they need to use all of these different materials correctly? In an effort to redirect the conversation back to evidence-backed science, Dr Garg has written an entire book on just that subject.
Setting the Record Straight
It had become impossible for clinicians to separate the science from the sell.
“Once we had the book out there,” Dr Garg recalls, “we stopped doing the CE courses. After we stopped lecturing on it, a lot of misinformation came in to take our place, and that led to a lot of confusion. I felt it was time for an update. I wanted to explain things in a way that would minimize the confusion among clinicians and reduce the effect of ‘paralysis by analysis’—where you overanalyze something so much that you get overwhelmed and never actually use it. The other issue was that commercial interests had completely taken over the conversation. It had become impossible for clinicians to separate the science from the sell. I wanted to get the information back on track.”
Autologous Blood Concentrates (Garg Multimedia Group, 2018) is the result of those efforts. With this book, Dr Garg has stepped back in as a strong, objective voice capable of helping to guide the present conversation surrounding autologous blood concentrates. In the spirit of setting aside his own personal interests as co-discoverer of PRP, he has even attempted to forego the term PRP and instead adopted autologous blood concentrates as a generic term for the materials, while also humbly admitting how hard the habit was to break:
“In this book,” he explains, “I’ve tried to minimize the chest pounding and salesmanship that has degraded the conversation into debates on nomenclature and instead focus on the science, biology, and clinical applications. I wanted the book to reach clinicians regardless of which machine or nomenclature they were most familiar with, so I didn’t want whichever acronym I used to be a roadblock for anyone. That’s why the title of the book is Autologous Blood Concentrates. I’ve tried to use that terminology as often as possible in the book. However, because I’ve called it PRP for 25 years, there are several cases where I’ve slipped and called it PRP. The reader is welcome to simply substitute ‘autologous blood concentrate’ in these instances; the information remains the same.
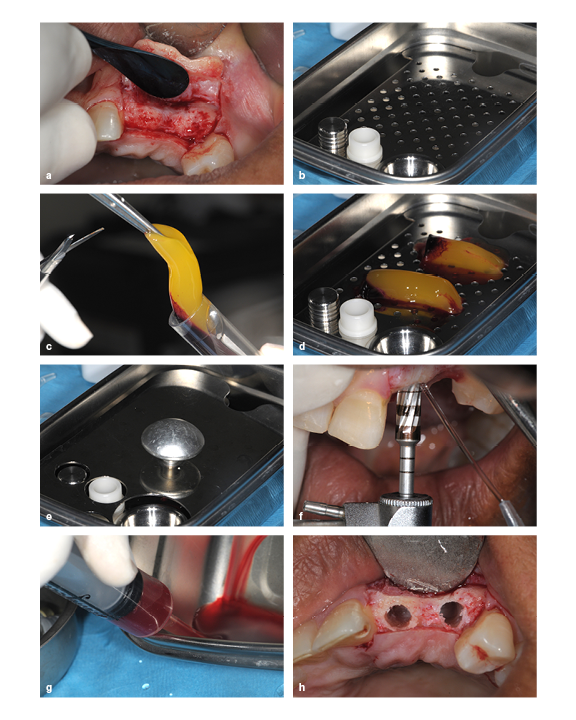
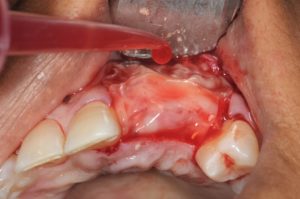
(a) The ridge is too thin for implant placement, so augmentation is required. (b) A PRP membrane kit. (c) PRP gel being removed from the test tube. (d and e) PRP gel placed in the membrane disk. (f) The ridge is widened by running the bur in reverse. (g) PRP exudate is extracted from the membrane dish using a syringe. (h) The prepared socket.
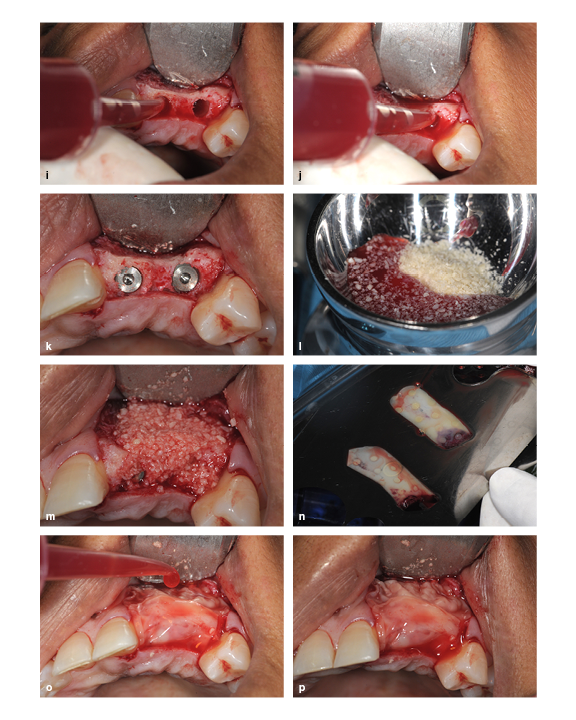
(i and j) The PRP exudate is used to hydrate the prepared socket. (k) Implant placement. (l) The freeze-dried bone allograft particles are hydrated with PRP exudate and mixed. (m) The ridge is packed with the bone graft particle and PRP exudate mixture. (n) The PRP membrane is uncovered in the membrane kit. (o) The PRP membrane is placed and then hydrated with PRP exudate. (p) The final view.
“This material has taken off,” he continues, “because it’s natural, it’s inexpensive, it’s safe, and it has so many applications. I wrote this book to make the information more accessible to clinicians, but I especially wanted to make the material itself accessible. While it must be remembered that it can’t make a bad surgery good, it will make a good surgery better, and that is what’s worthwhile.”
While it may not be made of Moroccan glass, Dr Garg’s book brings value to the field by redirecting the conversation of autologous blood concentrates out of the profit margins and back to the patient benefits. It represents a road map clinicians can use to navigate the plethora of available information on these materials and translate that information into clinical decision-making. Similarly, Dr Garg’s book provides a model for researchers on how these materials can be described and dealt with in the literature in order to minimize confusion as the field continues to evolve and press forward. In an industry that is still finding its footing with regard to communication, standardization, and replication of results, this book will certainly have a meaningful impact on the legacy of autologous blood concentrates.
 Arun K. Garg, DMD, is considered one of the world’s foremost authorities on bone biology, harvesting, and grafting for dental implant surgery. Alongside Dr Robert E. Marx, he pioneered the use of platelet-rich plasma (PRP). He received his dental degree from the University of Florida College of Dentistry in Gainesville, Florida, and completed his residency at the University of Miami Jackson Memorial Hospital in Miami, Florida. He went on to serve for almost two decades as Professor of Surgery in the Division of Oral and Maxillofacial Surgery and as Director of Residency Training at the University of Miami Leonard M. Miller School of Medicine, where he was frequently awarded faculty member of the year by his residents. Dr Garg has published 8 medical textbooks and over 150 journal articles is currently the president of the International Dental Implant Association. An international lecturer, he also maintains six private practices throughout southern Florida and is the founder of Implant Seminars, a leading dental continuing education company.
Arun K. Garg, DMD, is considered one of the world’s foremost authorities on bone biology, harvesting, and grafting for dental implant surgery. Alongside Dr Robert E. Marx, he pioneered the use of platelet-rich plasma (PRP). He received his dental degree from the University of Florida College of Dentistry in Gainesville, Florida, and completed his residency at the University of Miami Jackson Memorial Hospital in Miami, Florida. He went on to serve for almost two decades as Professor of Surgery in the Division of Oral and Maxillofacial Surgery and as Director of Residency Training at the University of Miami Leonard M. Miller School of Medicine, where he was frequently awarded faculty member of the year by his residents. Dr Garg has published 8 medical textbooks and over 150 journal articles is currently the president of the International Dental Implant Association. An international lecturer, he also maintains six private practices throughout southern Florida and is the founder of Implant Seminars, a leading dental continuing education company.
Arun K. Garg
Since the discovery of platelet-rich plasma (PRP) 25 years ago, interest in the use of autologous blood concentrates as adjuncts to surgical treatment has exploded. As more and more medically useful components of autologous blood concentrates have been identified, a host of unique acronyms such as PRF, CGF, PRGF, and more have surfaced, resulting in significant confusion among clinicians as to which material to use and when. Written by one of the original co-discoverers of PRP, this book tackles this issue of “too much information” by illuminating the science behind the clinical use of autologous blood concentrates as adjuncts to surgical treatment and helps to establish a foundation of practical knowledge for clinical use. The first part of the book summarizes the current literature from all aspects of medicine currently using autologous blood concentrates, showing both the possible applications as well as the limitations of these biologic materials. The second part of the book provides step-by-step instructions and richly illustrated treatment protocols for a number of applications for autologous blood concentrates specific to the practice of implantology and oral and maxillofacial surgery. Comprehensively researched and expertly written, this book is a must for clinicians who are just beginning to incorporate autologous blood concentrate treatment into their practice as well as experienced practitioners.
224 pp; 398 illus; ©2018; ISBN 978-0-99918-832-3 (B0007); US $199
This article was written by Caitlin Davis, Quintessence Publishing.
©2018–2019 BY QUINTESSENCE PUBLISHING CO, INC. PRINTING OF THIS DOCUMENT IS RESTRICTED TO PERSONAL USE ONLY. NO PART MAY BE REPRODUCED OR TRANSMITTED IN ANY FORM WITHOUT WRITTEN PERMISSION FROM THE PUBLISHER.



























 Altamiro Flávio, DDS, graduated from the Federal University of Goiás College of Dentistry in 1990 and went on to become a specialist in dental prostheses at the Federal University of Uberlândia in 1992. In 2010, he created a continuing education course called “A Smile for Each Face,” which included topics such as facial analysis, digital planning, dental anatomy, direct and indirect veneers, restorations, botulinum toxin, facial fillers, and viscosupplementation. He continues to teach courses like this all over the world, and he also teaches the specialization course in restorative dentistry at the Brazilian Association of Dentistry in Goiás. Dr Flávio is an accredited member of the Brazilian Society of Aesthetic Dentistry and a founder of the Brazilian Society of Botulinum Toxin and Facial Implants. He maintains a private practice in Goiânia, Brazil.
Altamiro Flávio, DDS, graduated from the Federal University of Goiás College of Dentistry in 1990 and went on to become a specialist in dental prostheses at the Federal University of Uberlândia in 1992. In 2010, he created a continuing education course called “A Smile for Each Face,” which included topics such as facial analysis, digital planning, dental anatomy, direct and indirect veneers, restorations, botulinum toxin, facial fillers, and viscosupplementation. He continues to teach courses like this all over the world, and he also teaches the specialization course in restorative dentistry at the Brazilian Association of Dentistry in Goiás. Dr Flávio is an accredited member of the Brazilian Society of Aesthetic Dentistry and a founder of the Brazilian Society of Botulinum Toxin and Facial Implants. He maintains a private practice in Goiânia, Brazil.